Santina Conte, MD1 and Monica K. Li, MD, FRCPC, FAAD2
1Division of Dermatology, McGill University, Montréal, QC, Canada
2Department of Dermatology and Skin Science, University of British Columbia, Vancouver, BC, Canada
Conflict of interest: Monica K. Li is a consultant and speaker for Galderma Canada. Santina Conte has no relevant conflicts of interest.
Funding sources: None.
Abstract:
Acne vulgaris, caused by pathophysiological processes at the pilosebaceous unit, is among the most common chronic dermatological disorders. Acne sequelae, including scarring and dyspigmentation, are common, and are often more distressing to patients than active acne lesions, reinforcing the importance of prevention and effective treatment. Trifarotene, a novel fourth generation retinoid selective for retinoid acid receptor gamma, is approved for the management of moderate-to-severe facial and truncal acne, with recent data supporting its efficacy in acne-induced hyperpigmentation. The purpose of this paper is to review treatment modalities for post-inflammatory hyperpigmentation and present trifarotene as a novel, evidence-based topical option.
Keywords: acne, retinoid, trifarotene, hyperpigmentation
Introduction
Acne vulgaris (AV) is one of the most common dermatological disorders, triggered by chronic inflammation of the sebaceous gland in the hair follicle.1 In addition to being notably common worldwide, with an estimated global prevalence of 9.38%, it occurs most frequently among adolescents, with 35-100% having acne at some point during their lives.2 In Canada, it is estimated that 9 in 10 adolescents struggle with the disease, with AV commonly persisting into adulthood.3,4 Acne-induced scarring and acne-induced dyspigmentation may persist long after the resolution of the primary lesions.5 The longer a patient with acne waits before starting an effective treatment, the greater the risk of sequelae development.5 Because acne is a common and chronic inflammatory condition that is frequently difficult to effectively manage, prevention of sequelae requires an assertive and sustainable treatment plan.6
AV and its sequelae play an important role on short-term and long-term self-perception and mental health, with psychosocial phenomena observed in affected individuals, including depression, suicidal ideation, anxiety, psychosomatic symptoms, pain, discomfort, embarrassment and social inhibition, which limit participation in daily and social activities and interpersonal relationships.7,8 However, effective treatment of AV has been shown to reduce and prevent the development of acne sequelae as well as improve patients’ self-esteem, obsessive-compulsiveness, shame, embarrassment, body image, social assertiveness and self-confidence, reinforcing the importance of targeted management.5,7
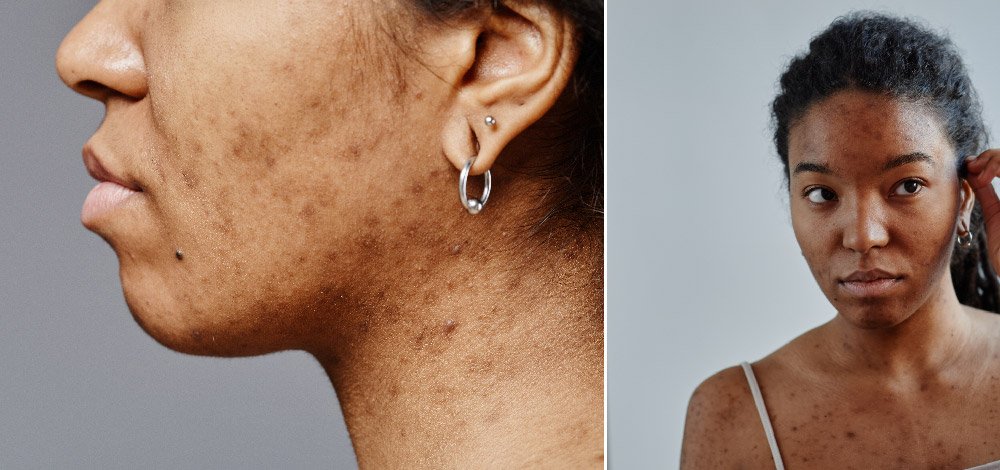
Acne-induced dyspigmentation is an impactful adverse sequela of AV in all skin phototypes, but is most frequent in skin of color. The chronic inflammation associated with AV may result in excess melanogenesis and abnormal melanin deposition, resulting in post-inflammatory hyperpigmentation (PIH) and post-inflammatory erythema (PIE) in all skin tones, with PIH more prevalent amongst patients with skin of color and PIE more common in lighter skin phototypes.9,10 Acne-induced hyperpigmentation (AIH) is often long-lasting with negative impacts on patients’ quality of life, frequently causing more distress than active acne lesions,9,11 and underlies their motivation to seek medical attention. In patients with active acne, the “post-inflammatory” in the term “PIH” may be misleading, as ongoing inflammation and new acne lesions present an additional challenge to treatment. Thus, in patients with active acne, it may be more accurate to refer to such pigmentary changes as AIH. For these patients, the most important goal in managing their AIH is ultimately optimal control and eventual resolution of their acne.
Currently, treatment options for acne-induced PIH can be grouped into four main classes: keratolytics, retinoids, corticosteroids, and depigmenting agents.12 Topical therapy with hydroquinone is considered to be the gold standard treatment for hyperpigmentation, but usage may be limited due to adverse effects such as exogenous ochronosis.13,14 The current therapeutic approach for PIH secondary to melasma specifically includes triple topical combination therapy with hydroquinone, a retinoid and a corticosteroid, but outcomes are often suboptimal due to adverse effects, limited efficacy and post-treatment relapses.15 Recently, a phase 4 doubleblind, placebo-controlled study by Alexis et al. demonstrated that trifarotene, a selective fourth generation retinoid, led to rapid improvement in overall disease severity, improvement in post-AV hyperpigmentation index, high patient satisfaction, and favorable treatment compliance, supporting the use of this topical retinoid in the management of AV-induced PIH.16
Background
Trifarotene is a new fourth generation topical retinoid approved by Health Canada and the United States Food and Drug Administration for the treatment of AV in patients over the ages of 12 years in Canada and 9 years in the United States.17,18 In Canada, the indication is for topical treatment of AV of the face and/or trunk.17 It is sold as a 0.005% or 50 μg/g cream (Aklief® cream) in 75 g pumps, and is to be applied to affected areas once daily.19 Relevant ingredients include allantoin, copolymer of acrylamide and sodium acryloyldimethyltaurate with isohexadecane, polysorbate 80, sorbitan oleate, cyclomethicone 5, ethanol, phenoxyethanol, propylene glycol, purified water and medium-chain triglycerides, which have been noted to promote the proliferation of healthy tissue, wound healing, emulsification, improved moisturization and enhanced epithelial keratinocyte turnover, resulting in short-term thinning of the stratum corneum and resolution/prevention of comedones, as well as long-term thickening of the epithelium.17,20-22
Retinoid acid receptor gamma (RAR-γ) is the principal receptor subtype found in the epidermis and is targeted by trifarotene.23,24 Notably, acne-induced PIH occurs primarily in the epidermis.25 Trifarotene, similar to retinoids from previous generations, normalizes follicular keratinization and has anti-inflammatory effects by modifying the expression of retinoic acid receptorregulated genes.26 Moreover, a recent mouse study comparing the activity of several retinoids found that trifarotene demonstrated superior depigmenting and anti-pigmenting properties on mouse tail skin with and without ultraviolet (UV) exposure, reinforcing its favorability in the management of AV-induced PIH.27
Supporting Evidence from Clinical Trials
Results from a Phase 4 Study
In a phase 4, double-blind, parallel-group study of patients (n=123) aged 13 to 35 years with moderate AV and AV-induced hyperpigmentation, the efficacy and safety of trifarotene 50 mcg/g applied once daily in conjunction with a skin care regimen (Cetaphil® Moisturizing Lotion, Cetaphil® Gentle Skin Cleanser and Cetaphil® PRO DermaControl Oil Control Moisturizer SPF30 sunscreen) over the course of 24 weeks was assessed.16 Notably, patients in the vehicle arm also used daily sun protection. Trifarotene’s efficacy in the management of AV-induced pigmentation was determined through overall disease severity scores (ODS) and post-AV hyperpigmentation indices (PAHPI). Moderate AV was defined as an Investigator Global Assessment (IGA) score of 3 on the face, ≥20 inflammatory lesions, and ≥25 non-inflammatory lesions (excluding the nose). Moderate to marked acne-induced hyperpigmentation (AIH) was qualified using an overall disease severity hyperpigmentation scale, with included patients having a score between 4-6. Patients with greater than one AV nodule or any number of cysts were excluded from the study, as well as female patients who were pregnant, lactating or using oral contraceptives approved for AV treatment. The primary endpoint was absolute change from baseline in ODS at 24 weeks of treatment. Additionally, percent change of AIH from baseline to week 24, absolute/percent change in AIH overall disease severity scores at weeks 12, 16 and 20, average AIH lesion size, post-AV hyperpigmentation index scores and intensity were assessed as secondary AIH variables. AV-related outcomes evaluated included absolute and percent change in total, inflammatory and non-inflammatory lesions, in addition to the proportion of patients achieving IGA success at 12 and 24 weeks. Subjective outcomes were assessed by means of qualitative exit interviews and a treatment satisfaction questionnaire.
The PAHPI score, a secondary endpoint measured in this trial, represents a real-world reflection and should be considered by all clinicians as a quantifiable, reproducible way to measure disease-related concerns such as symptom severity and the impact of skin disease on patients’ quality of life.28 More specifically, it is a composite scale and includes quantification of the number, size and intensity of acne lesions.16 With regards to ODS, there was a statistically significant reduction in pigmentation with trifarotene as compared to the control at 12 weeks, however significance was not achieved at weeks 16 and 24. Overall, ODS decreased by -45.4% in the trifarotene group and -44.9% in the vehicle group, while the decrease in the percentage of patients with marked AIH at 12 weeks was much more impressive in the trifarotene group as compared to the vehicle (trifarotene -26.3%, vehicle -2%). Both the treatment and vehicle groups showing improvement at 6 months may be reflective of the natural rate of pigment clearance from the skin. For PAHPI scoring, statistically significant reductions in size, intensity and number of hyperpigmented lesions were observed in patients treated with trifarotene as compared to the control group at weeks 20 and 24, while sub-scores assessing size, intensity and number of lesions showed higher absolute and percent change in the trifarotene group as compared to the vehicle-treated group. Overall, there were greater reductions in PAHPI total facial scores in the trifarotene group (-8.4% at 12 weeks, -18.9% at 24 weeks) than the control group (-4.5% at 12 weeks, -11.3% at 24 weeks), with PAHPI sub-scores in the treatment group being nearly double those of patients treated with the vehicle. When determining improvement in AIH, over 60% of patients were noted to have lighter or much lighter skin when treated with trifarotene over 24 weeks, while the number of patients with over 45 AIH lesions decreased more significantly amongst treated patients than those who received the vehicle formulation (trifarotene -14.7%, vehicle -1.3%). Both subjects (trifarotene 91.2%, vehicle 83%) and investigators (trifarotene 91.2%, vehicle 81.1%) noted slightly better improvements in AIH with trifarotene as compared to the vehicle, which was also appreciated upon review of patients’ photographs.
In addition to assessing trifarotene’s efficacy in the management of AV-induced hyperpigmentation, changes in AV lesions were also evaluated. Trifarotene was found to result in significantly greater reductions in total acne lesion counts than the vehicle (12 weeks: trifarotene -64.1%, vehicle -46.7%; 24 weeks: trifarotene -72.0%, vehicle -62.8%). IGA success was also more notable among patients treated with trifarotene, whereby 38.0% achieved IGA success by week 12 as compared to 20.8% of vehicle-treated patients, with continued improvement through to 24 weeks (trifarotene 61.1%, vehicle 39.4%).
To evaluate patients’ perspectives with regards to the efficacy of treatment, exit interviews were performed (n=30, mean age 24.8 years, 73.3% Fitzpatrick skin type IV-VI). Patients treated with trifarotene reported a greater reduction in AIH severity from baseline to week 24, with a higher proportion of individuals in the trifarotene group reporting an improvement in AIH (100%) compared to the vehicle group (83%). Moreover, the only patients to perceive a stagnation or worsening of their AIH were in the vehicle group. Furthermore, significantly more patients treated with trifarotene reported that their AIH was “much better” (83.3%) when compared to control patients (61.1%).
On safety, more adverse events were noted in vehicle-treated patients (n=19, 30.2%) than those treated with trifarotene (n=10, 16.7%), with differences thought to be secondary to infections, including COVID-19. Notably, the study was conducted during the height of the pandemic. Two vehicle-treated patients reported burning and dry skin at the application site, and all adverse events were mild or moderate in severity. Both treatments were shown to have good local tolerability, which could be due to the Cleanse, Treat, Moisturize, Protect (CTMP) regimen mandated on all patients in the clinical study.
This phase 4 study had multiple strengths. First, over two-thirds of all subjects included in the study were of Fitzpatrick phototypes IV to VI (67.5%), with 36.7% of patients treated with trifarotene of African American descent. Given that PIH is long-lasting, highly distressing and disproportionately affects individuals with skin phototypes III-VI, inclusion of patients with darker phototypes in clinical trials supports improved real-world translation.29,30 Secondly, whereas traditional acne treatment clinical trials usually span 3 months, this trial assesses trifarotene’s efficacy over 6 months, providing greater insight into the drug’s ability to alleviate inflammatory changes in the skin and prevent and/or improve acne-induced pigmentary outcomes.
Short Summary of Results from Phase 4 Study
In essence, Alexis et al.’s clinical trial demonstrates that trifarotene is highly effective with good tolerability in the management of AV and AV-induced hyperpigmentation when used in conjunction with an appropriate skin care regimen that includes UV protection, yielding changes in overall disease severity and post-acne hyperpigmentation indices as early as 12 weeks. All parameters were noted to more significantly improve with trifarotene in comparison to vehicle therapy, including AIH lesion size, intensity and number, as well as total number of AV lesions, including both inflammatory and non-inflammatory lesions, utilizing assessments such as IGA, ODS score, post-AV hyperpigmentation index, exit interviews and photography.
Other Treatment Modalities for Post-Inflammatory Hyperpigmentation
There are currently several available treatment modalities for the management of AV-induced PIH. Regardless of the plethora of options, long-term strategies should include UV protection with sunscreen application of sun protection factor (SPF) 30 or above, a consistent skin care routine and where possible, the use of a topical retinoid proven to have depigmenting effects. Moreover, PIH has been shown to persist for greater than 1 year in nearly 50% of cases, and for over 5 years in 22.3% of patients affected by acne.31 Recognizing the need for prolonged treatment, consistent patient education is necessary to ensure long-term commitment and adherence to proposed treatment modalities, as well as to set patient expectations.32
With management of PIH, treating the underlying, causative inflammatory process is the first step. In the case of AIH, trifarotene’s potent anti-inflammatory activity may contribute to its clinically demonstrated ability to limit the severity and duration of PIH.24 Other safe and well-tolerated topicals with cutaneous anti-inflammatory properties include dapsone and clindamycin phosphate gels, which were also found to decrease acne severity and PIH.33,34 Additionally, tyrosinase inhibitors, namely hydroquinone, are the mainstay of PIH therapy and work by suppressing melanin production.32 Cysteamine cream has also been proven to have comparable efficacy to topical hydroquinone. Should a less irritating agent be needed or desired, mequinol can be tried.13 Currently, PIH therapies have been best studied in melasma, with triple therapy preferred over unimodal therapy, such as combining hydroquinone 4%, tretinoin 0.05% and fluocinolone acetonide 0.01%.32 The hypothetical rationale of triple combination therapy is that the three molecules work synergistically to interfere with the production of melanin, slow the transfer of melanin to melanosomes, and accelerate the clearance of melanin from the epidermis by increasing keratinocyte turnover. Moreover, the retinoid counters the risk of steroid-induced skin atrophy, while the steroid component counters the irritation caused by the other two ingredients, which could lead to both patient discomfort and be counterproductive by inducing PIH. However, caution must be exercised, ensuring that patients understand the risks of exogenous ochronosis and steroid-induced cutaneous changes with prolonged and continuous hydroquinone and topical corticosteroid use, respectively. The usage of chemical peels, which work by removing the epidermal cells containing excess melanin, have been proven to be efficacious, with the most common peels using glycolic, salicylic and trichloroacetic acids.32 A comparative study previously demonstrated that serial glycolic acid peels with a modified Kligman formula (hydroquinone 2%, tretinoin 0.05% and hydrocortisone 1%) were efficacious and safe in the treatment of facial PIH in dark-skinned patients, while topical azelaic acid 15% gel was also found to successfully reduce acne and PIH in patients with skin of color.35,36 Laser therapy has also proven to be successful in the management of PIH, including neodymium-doped yttrium aluminum garnet (Nd:YAG), picosecond (short, intense pulses) and ruby lasers.12,32 However, as laser modalities deliver thermal energy, which may drive additional hyperpigmentation, usage should be reserved for experienced clinicians. Finally, regardless of the choice of therapy, any interventions must be well-tolerated and not add irritation or excess inflammation, given that the management of PIH is often lengthy and requires strict treatment adherence to optimize overall outcomes. Thus, user-friendly, well-tolerated and effective topical therapies, such as retinoids, are fundamental in the effective and sustainable management of acne-induced PIH.
Conclusion
Trifarotene 0.005% cream has been proven to be effective and welltolerated in the management of moderate AV and acne-induced PIH, likely due to its capacity to reduce inflammation throughout the epidermis via interactions with specific receptor isotypes. Not only has trifarotene proven to reduce the number and severity of active AV lesions, it has also shown to play a role in the prevention and reduction of PIH, which is especially important in preventing the long-term sequelae of acne in individuals of skin of color. Given its safety and tolerability profiles, as well as its relative cost-effectiveness, trifarotene should be considered when treating both acne and hyperpigmentation. Further studies evaluating the combination effect of trifarotene with other mainstay therapies, including a CTMP framework, beyond 6 months will be valuable in enhancing our understanding of optimal multimodal management of acne-induced hyperpigmentation.
Acknowledgement: We thank JP York, PhD and Rajeev Chavda, MBBS, MD, DBM for their editorial review and support.
References
- Lei Y, Jiang W, Peng C, et al. Advances in polymeric nano-delivery systems targeting hair follicles for the treatment of acne. Drug Deliv. 2024 Dec;31(1):2372269.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020 Apr 1;10(1):5754.
- Barbieri JS, Fulton R, Neergaard R, et al. Patient perspectives on the lived experience of acne and its treatment among adult women with acne: a qualitative study. JAMA Dermatol. 2021 Sep 1;157(9):1040-6.
- ACD chronique des experts | CDA expert series – Dr Jérôme Coulombe au sujet de l’acné. Video by Canadian Dermatology Association. Available from: https://dermatology.ca/public-patients/skin/acne/
- Layton A, Alexis A, Baldwin H, et al. Identifying gaps and providing recommendations to address shortcomings in the investigation of acne sequelae by the Personalising Acne: Consensus of Experts panel. JAAD Int. 2021 Aug 17;5:41-8.
- Yan Q, Zhang F, Qiao Z, et al. Investigating the mechanism of PAD in the treatment of acne based on network pharmacology and molecular docking: a review. Medicine (Baltimore). 2024 Jul 19;103(29):e38785.
- Tan JK. Psychosocial impact of acne vulgaris: evaluating the evidence. Skin Therapy Lett. 2004 Aug-Sep;9(7):1-3, 9.
- Hazarika N, Archana M. The psychosocial impact of acne vulgaris. Indian
J Dermatol. 2016 Sep-Oct;61(5):515-20. - Elbuluk N, Grimes P, Chien A, et al. The pathogenesis and management of acne-induced post-inflammatory hyperpigmentation. Am J Clin Dermatol. 2021 Nov;22(6):829-36.
- Cruz S, Vecerek N, Elbuluk N. Targeting inflammation in acne: current treatments and future prospects. Am J Clin Dermatol. 2023 Sep;24(5):681-94.
- Taylor S, Elbuluk N, Grimes P, et al. Treatment recommendations for acne-associated hyperpigmentation: results of the Delphi consensus process and a literature review. J Am Acad Dermatol. 2023 Aug;89(2):316-23.
- Zawar VP, Agarwal M, Vasudevan B. Treatment of postinflammatory pigmentation due to acne with q-switched neodymium-doped yttrium aluminum garnet in 78 Indian cases. J Cutan Aesthet Surg. 2015 Oct-Dec;8(4):222-6.
- Ahmadi K, Miri A, Bizaval Z, et al. Assessing the effectiveness of stabilized cysteamine 5% cream compared to hydroquinone 4%/ascorbic acid 3% combination cream in treating acne-induced post-inflammatory hyperpigmentation: a randomized, controlled study. J Clin Aesthet Dermatol. 2024 Apr;17(4):37-41.
- Narayanan D, Tyring SK. Hyperpigmented macules and patches on the face: exogenous ochronosis or lichen planus pigmentosus? J Drugs Dermatol. 2024 Jul 1;23(7):567-8.
- Mahajan VK, Patil A, Blicharz L, et al. Medical therapies for melasma. J Cosmet Dermatol. 2022 Sep;21(9):3707-28.
- Alexis A, Del Rosso JQ, Forman S, et al. Importance of treating acne sequelae in skin of color: 6-month phase IV study of trifarotene with an appropriate skincare routine including UV protection in acne-induced postinflammatory hyperpigmentation. Int J Dermatol. 2024 Jun;63(6):806-15.
- AKLIEF™ (trifarotene) cream. Date of preparation: November 25, 2019. Galderma Canada Inc., Thornhill, ON.
- Stewart J. Aklief FDA approval history. Drugs.com. [updated 27 January 2021]. Available from: https://www.drugs.com/history/aklief. html#:~:text=Aklief%20%20FDA%20a%20Pproval%20history
- UpToDate. Trifarotene: drug information. 2024. In: UpToDate [Internet]. Available from: https://www.uptodate.com/contents/trifarotene-drug-information?search=aklief&topicRef=42&source=see_link
- Araújo LU, Grabe-Guimarães A, Mosqueira VC, et al. Profile of wound healing process induced by allantoin. Acta Cir Bras. 2010 Oct;25(5):460-6.
- Al Jasser M, Mebuke N, de Gannes GC. Propylene glycol: an often unrecognized cause of allergic contact dermatitis in patients using topical corticosteroids. Skin Therapy Lett. 2011 May;16(5):5-7.
- Asarch A, Scheinman PL. Sorbitan sesquioleate, a common emulsifier in topical corticosteroids, is an important contact allergen. Dermatitis. 2008 Nov-Dec;19(6):323-7.
- Dreno B, Chavda R, Julia V, et al. Transcriptomics analysis indicates trifarotene reverses acne-related gene expression changes. Front Med (Lausanne). 2021 Oct 22;8:745822.
- Brumfiel CM, Patel MH, Bell KA, et al. Assessing the safety and efficacy of trifarotene in the treatment of acne vulgaris. Ther Clin Risk Manag. 2021 Jul 26;17:755-63.
- Silpa-Archa N, Kohli I, Chaowattanapanit S, et al. Postinflammatory hyperpigmentation: a comprehensive overview: epidemiology, pathogenesis, clinical presentation, and noninvasive assessment technique. J Am Acad Dermatol. 2017 Oct;77(4):591-605.
- Osayande A, McGraw-Senat CM. Trifarotene (Aklief) for the treatment of acne. Am Fam Physician. 2020 Oct 15;102(8):499-504.
- Aubert J, Piwnica D, Bertino B, et al. Nonclinical and human pharmacology of the potent and selective topical retinoic acid receptor-γ agonist trifarotene. Br J Dermatol. 2018 Aug;179(2):442-56.
- Snyder AM, Chen SC, Chren MM, et al.; Dermatology PRO Consortium. Patient-reported outcome measures and their clinical applications in dermatology. Am J Clin Dermatol. 2023 Jul;24(4):499-511.
- Manjaly P, Kamal K, Ly S, et al. Disparities in state Medicaid coverage of tretinoin for pigmentary disorders compared to acne vulgaris. J Drugs Dermatol. 2024 Jun 1;23(6):e151-3.
- Hossain OB, Labiak A, Mieczkowska K, et al. Postinflammatory hyperpigmentation following Mohs micrographic surgery: an observational study. J Drugs Dermatol. 2024 May 1;23(5):316-21.
- Abad-Casintahan F, Chow SK, Goh CL, et al; Asian Acne Board. Frequency and characteristics of acne-related post-inflammatory hyperpigmentation. J Dermatol. 2016 Jul;43(7):826-8.
- Lawrence E, Al Aboud KM. Postinflammatory hyperpigmentation. 2022 Oct 3. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan.
- El-Kashlan N, Cices A, Kaufman B, et al. An open-label study to investigate the efficacy and tolerability of dapsone gel, 7.5% in the treatment of acne vulgaris in men and women with skin of color. J Drugs Dermatol. 2024 Jun 1;23(6):410-7.
- Callender VD, Young CM, Kindred C, et al. Efficacy and safety of clindamycin phosphate 1.2% and tretinoin 0.025% gel for the treatment of acne and acne-induced post-inflammatory hyperpigmentation in patients with skin of color. J Clin Aesthet Dermatol. 2012 Jul;5(7):25-32.
- Sarkar R, Parmar NV, Kapoor S. Treatment of postinflammatory hyperpigmentation with a combination of glycolic acid peels and a topical regimen in dark-skinned patients: a comparative study. Dermatol Surg. 2017 Apr;43(4):566-73.
- Kircik LH. Efficacy and safety of azelaic acid (AzA) gel 15% in the treatment of post-inflammatory hyperpigmentation and acne: a 16-week, baseline-controlled study. J Drugs Dermatol. 2011 Jun;10(6):586-90.